Background: The new standard of care for fit patients with refractory or early relapse of diffuse large B-cell lymphoma (DLBCL) is chimeric antigen receptor T-cell (CAR-T) therapy. However, for patients with a relapse ≥12 months after completing frontline therapy, salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) remains the standard of care. There is a need to characterize such patients and their survival in view of the recent shift in treatment paradigm.
Methods: Patients with DLBCL that relapsed ≥12 months after R-CHOP or R-CHOP-like frontline therapy who underwent salvage therapy and ASCT at Mayo Clinic or University of Iowa between 07/2000 and 4/2020 were identified from institutional lymphoma and transplant databases. Clinical characteristics, treatment information, and outcome data were abstracted. Progression-free survival (PFS) and overall survival (OS) from the time of ASCT were analyzed using Kaplan-Meier method and Cox proportional hazards models. Statistical analyses were performed in JMP v15.
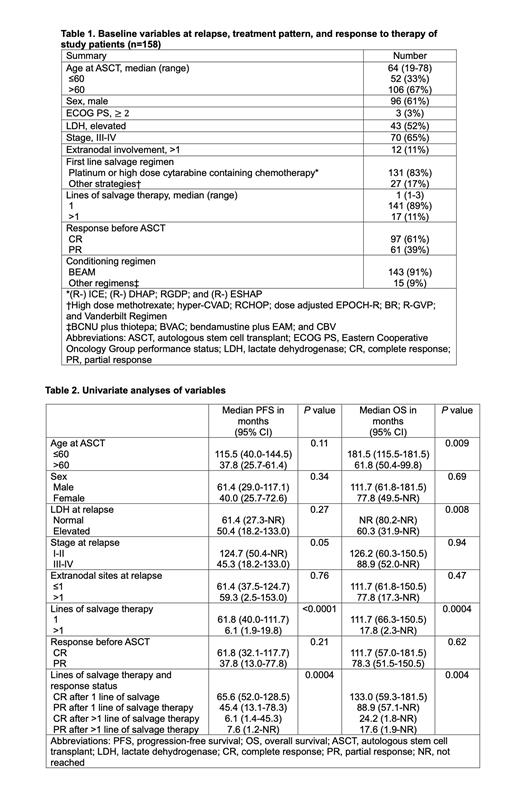
Results: A total of 158 patients with late relapsed DLBCL who underwent salvage chemotherapy and ASCT were identified. Baseline characteristics at relapse/ASCT are shown in Table 1. Median time from frontline therapy completion to 1st relapse was 26.4 months (range 12.0-152.4). Median age at relapse was 63 years (range 19-77), and 96 (61%) patients were male. A minority (3; 3%) had ECOG PS ≥2. 43 (52%) patients had an elevated serum LDH level, 70 (65%) had advanced stage disease, and 12 (11%) had >1 extranodal involvement.
Median line of salvage therapy was 1 (range 1-3), and 17 (11%) patients required >1 line of salvage therapy. Best response before ASCT was complete response (CR) in 97 (61%) and partial response (PR) in 61 (39%). Median age at ASCT was 64 years (range 19-78). Median follow-up after ASCT was 91.5 months (95% CI 74.0-103.3). Median PFS and OS were 54.5 (95% CI 31.9-77.8) and 99.8 (95% CI 60.3-144.5) months, respectively. The 2-year PFS and OS rates were 64% (95% CI 56-71) and 81% (95% CI 74-87), respectively. No statistically significant difference in PFS was seen based on age at ASCT, sex, serum LDH, stage, or extranodal site involvement of >1 at relapse (Table 2). However, patients who required > 1 line of salvage therapy, compared to those requiring 1 line of salvage therapy, had significantly inferior PFS (median 6.1 vs 61.8 months, P <0.0001) and OS (17.8 vs 111.7 months, P <0.0004). There was no statistically significant difference in survival in patients who achieved CR vs PR prior to ASCT, with a median PFS of 61.8 vs 37.8 months( P=0.21) and a median OS of 111.7 vs 78.3 months ( P=0.62). Patients who achieved CR after 1 line of salvage therapy had the most favorable PFS and OS, with a median PFS of 65.6 vs 45.4 vs 6.1 vs 7.6 months ( P=0.0004) and a median OS of 133.0 vs 88.9 vs 24.2 vs 17.6 months ( P=0.004) in patients achieving CR after 1 line of salvage therapy vs PR after 1 line of salvage therapy vs CR after >1 line of salvage therapy vs PR after >1 line of salvage therapy, respectively (Table 2).
In multivariate Cox regression models adjusted for age at ASCT and sex, patients requiring > 1 line of salvage therapy, compared to those who required 1 line of salvage therapy, had significantly inferior PFS with a hazard ratio (HR) of 3.25 (95% CI 1.82-5.78, P <0.0001) and OS with a HR of 3.50 (95% CI 1.86-6.60, P=0.0001). However, there remained no significant difference in survival based on response status (CR vs PR) with a HR for PFS 0.78 (95% CI 0.52-1.17, P=0.23) and OS 0.93 (95% CI 0.58-1.47, P=0.74).
Conclusions: Survival after ASCT was excellent in patients with late relapsed DLBCL achieving CR after 1 line of salvage chemotherapy. Favorable survival outcomes were seen in patients who achieved PR after 1 line of salvage therapy. These data support the current clinical practice of ASCT consolidation in these patients. However, post-ASCT survival was poor in patients who required more than 1 line of salvage chemotherapy, despite achieving a satisfactory response to subsequent lines of salvage therapy. Alternative treatment strategies such as CAR-T therapy should be considered in such patients.
Disclosures
Wang:Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; Morphosys: Research Funding; Genentech: Research Funding; Novartis: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Paludo:Biofourmis: Research Funding; Karyopharm: Research Funding; AbbVie: Consultancy. Ansell:ADC Therapeutics: Other: Contracted Research; Affirmed: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research. Nowakowski:Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Kite Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Selvita Inc: Consultancy; TG Therapeutics: Consultancy; Celgene Corporation: Consultancy; ADC Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; MEI Pharma: Consultancy; Incyte: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Seagen: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kymera Therapeutics: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy; Zai Lab Limited: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Farooq:MorphoSys: Consultancy; Kite, a Gilead Company: Honoraria; Caribou: Consultancy, Honoraria; Regeneron: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal